Plan to join us for the ISPE Canada Education and Product Symposium on May 8 – 9, 2023. The theme of this year’s program is Advancing Canadian Pharma and Biopharma Manufacturing in the “New Normal”.
To ensure product quality and supply chain safety, improving manufacturing efficiency is a key initiative for ISPE while always putting the patient first. Our industry makes products that are life-changing, life-sustaining, and impact hundreds of millions of people worldwide.
The challenges facing the pharmaceutical manufacturing industry have increased with economic and regulatory pressures, technology changes and escalating cost. The way we approach solutions needs to be innovative in order to keep pace with today’s demands.
SYMPOSIUM AGENDA MONDAY, May 08, 2023
7:00 AM to 8:45 AM
FOYER – 11th Floor
Registration
7:45 AM to 8:45 AM
Montreal B & C – 11th Floor
Continental Breakfast & Product Show
8:55 AM to 10:15 AM
Montreal A – 11th Floor (keynote)
Keynote Session
 Health Canada’s Lessons Learned from the COVID-19 Pandemic
Health Canada’s Lessons Learned from the COVID-19 PandemicSpeakers
10:15 AM to 11:00 AM
Montreal B & C – 11th Floor
Break & Product Show
11:00 AM to 12:00 PM
St. Jacques – 3rd Floor
Applying Lean Six Sigma to Navigating Supply Chain Complexity during the Pandemic Era
Kossi MolleyLSSBB Sr. Manager, Manufacturing & Operational Excellence Mivado Global Performance
11:00 AM to 12:00 PM
Riopelle – 3rd Floor
The Challenges of Qualifying Ancillary / Raw Materials for Clinical Use in the Cell & Gene Therapy Market
Lynn CsontosVice President, Regulatory Affairs STEMCELL Technologies
11:00 AM to 12:00 PM
Victoria – 3rd Floor
ROADMAP TO ECM (ENGINEERING CHANGE MANAGEMENT)
12:00 PM to 1:30 PM
Montreal B & C – 11th Floor
Lunch & Product Show
13:30 PM to 14:30 PM
St. Jacques – 3rd Floor
A New Generation of Therapeutics with New Challenges in the New Normal – Latest Developments in Aseptic Filling for Cell and Gene Therapies
13:30 PM to 14:30 PM
Riopelle – 3rd Floor
PROCESS VALIDATION = QBD + PPQ + CPV
13:30 PM to 14:30 PM
Victoria – 3rd Floor
Lessons learned from commissioning a recently renovated facility
Jamie EvansSenior Process Engineer, Utilities Grifols
Ester SanchezProject Manager Grifols
14:30 PM to 15:30 PM
St. Jacques – 3rd Floor
Ensuring Drug Supply Through Digital Supply Chain Enablement and Solutions
Zaki KhanDirector, IT, Commercial Digital Solutions Thermo Fisher Scientific
Noella Dallaire (she/her)Sr. Mgr. Portfolio Services Thermo Fisher Scientific
14:30 PM to 15:30 PM
Riopelle – 3rd Floor
Cleaning Validation of a Multi Product Facility – A Risk Based Approach
Roshan ShahSenior Validation Manager Hyde Engineering & Consulting
14:30 PM to 15:30 PM
Victoria – 3rd Floor
How to Navigate the Pharma 4.0 wave from a compliance perspective
Jovana NakicHead of Customer Onboarding and Professional Services GENAIZ/Groupe Uni3T
15:30 PM to 16:30 PM
Montreal B & C – 11th Floor
Break & Product Show
16:30 PM to 17:30 PM
St. Jacques – 3rd Floor
The Unique Challenges of Cell and Gene Therapies vs Biologics Manufacturing (And Where We Are Not That Different)!
Gayle PiatDirector, Alberta Cell Therapy Manufacturing (ACTM)
16:30 PM to 17:30 PM
Riopelle – 3rd Floor
Introduction to Pharmaceutical Sustainability: Clean Utility Case Study
Jeff BarnesManaging Director Stilmas America
Paolo LeaniTechnical Director Stilmas Americas
16:30 PM to 17:30 PM
Victoria – 3rd Floor
Data Integrity & Cloud-Based Systems
Fatou Pompilus – Touré, ing.Validation Engineer Laporte Consultants
17:30 PM to 19:00 PM
Foyer – 11th Floor
Cocktails & Appetizers
19:00 PM to 21:00 PM
Montreal B & C – 11th Floor
Dinner
21:00 PM Onwards
To be announced
SYMPOSIUM AGENDA Tuesday, May 09, 2023
7:00 AM to 8:15 AM
Montreal B & C – 11th Floor
Continental Breakfast & Product Show
08:15 AM to 09:15 AM
St. Jacques – 3rd Floor
Trends in ESG Reporting and Digital Transformation
Kevin McKeeVice President, Global ESG Solutions Lead WSP, Digital EHS & ESG Solutions
08:15 AM to 09:15 AM
Victoria – 3rd Floor
Modernizing a legacy facility with Product and Equipment context and AI/ML capability for Reliability and Optimization.
Mathew D’souzaHead of Site Asset Management, Engineering Technical Services Sanofi”
09:15 AM to 10:30 AM
Montreal B & C – 11th Floor
Break & Product Show
10:30 AM to 11:30 AM
St. Jacques – 3rd Floor
Advancing towards sustainability in pharmaceutical manufacturing
MELANEE SHORTDIRECTOR, SUSTAINABLE INFRASTRUCTUR
Chris Fitzgibbon Process Engineer Cheme Engineering
Gregory Wright Project Manager Cheme Engineering
10:30 AM to 11:30 AM
Victoria – 3rd Floor
Best Practices for major retrofit implementation in Biotech site with ongoing commercial cGMP activities.
Aqeel MonpuriSenior Manager Capital Project and Portfolio Resilience Biotechnology Inc
11:30 AM to 12:30 PM
Montreal C – 11th Floor (keynote)
Healthy Work Culture in a Hybrid World
Lynn Csontos (she/her)Vice President, Regulatory Affairs STEMCELL Technologies
Nicole Cosmopoulos (she/her)Vice President – HR Precision NanoSystems
Benoit Lemelin (he/him)North America People & Culture, PBP Canada Head Sanofi
Stephanie Michaud (she/her)CEO & President BioCanRx
12:30 PM to 12:45 PM
Montreal B & C – 11th Floor
Closing Ceremony
13:00 PM to 16:00 PM
Montreal B & C or one of the breakout rooms
Board Meeting with Lunch included
Symposium Speakers
EVENT LOCATION

Le Westin Hotel
270 Saint-Antoine St. W., Montreal, Quebec H2Y 0A3
514-380-3333
ISPE Canada has an excellent preferred room rate of $282.00 CND…don’t miss out on this rate and book now! The group rate for reservations 3 days pre and post contracted dates upon availability.
(Complimentary internet in all guestrooms & complimentary basic WIFI in the general session & exhibit/meal room for all attendees)
Le Westin Montreal is conveniently located at the junction of Old Montreal and downtown in beautiful, Montreal, Canada. Le Westin Montreal features the unparalleled comfort of Westin’s Heavenly bed, in-room safe, iHome with mp3 clock, small fridge, 24-hour fitness centre, indoor pool and sauna. The hotel is connected by underground walkway to Montreal Palais des Congres (main Convention Centre) and to Montreal Subway (Metro).
Take advantage of the special group rate for 2023 Annual ISPE Canada Education & Product Symposium: $282 CAD per night (includes free internet access)
Special group rate offered until 7:00 am Tuesday, April 18, 2023
Take advantage of a group rate of $282 through the online reservation link below or call 1-866-837-4262 (mention the ISPE group rate of $282 per night):
Book your group rate for ISPE Education & Product Symposium
Book now as this amazing rate at this great hotel is limited.
ATTENDEE REGISTRATION
Attendee passes include access to exhibits, educational sessions and all meals including Monday evening dinner
| Registration Category | Fee |
|---|---|
| Members | $675.00 |
| Non-members | $800.00 |
| Group (5+) at $650 each (membership not required) | $3,250.00 |
| Young Professionals (ISPE Membership Required) | $350.00 |
| Students (ISPE Membership Required) | $50 |
Register Now for the Symposium
EXHIBITORS REGISTRATION
| Exhibition Level | Fee |
|---|---|
| 10 × 10 Booth Exhibitor ( two attendees ) |
$3,500 |
| TableTop Exhibitor ( one attendee ) |
$2,000 |
Additional charges will apply for passport program and power-drop
| Program | Fee |
|---|---|
| Passport Program (unique opportunity to interact with attendees) | $300 |
| Power-drop (standard power cord included with exhibit package) | Contact Dina Iezzi at info@ispecanada.org |
ISPE Canada Education and Product Symposium - May 8 - 9, 2023
ISPE Canada Education and Product Symposium - May 8 - 9, 2023
SPONSORS REGISTRATION
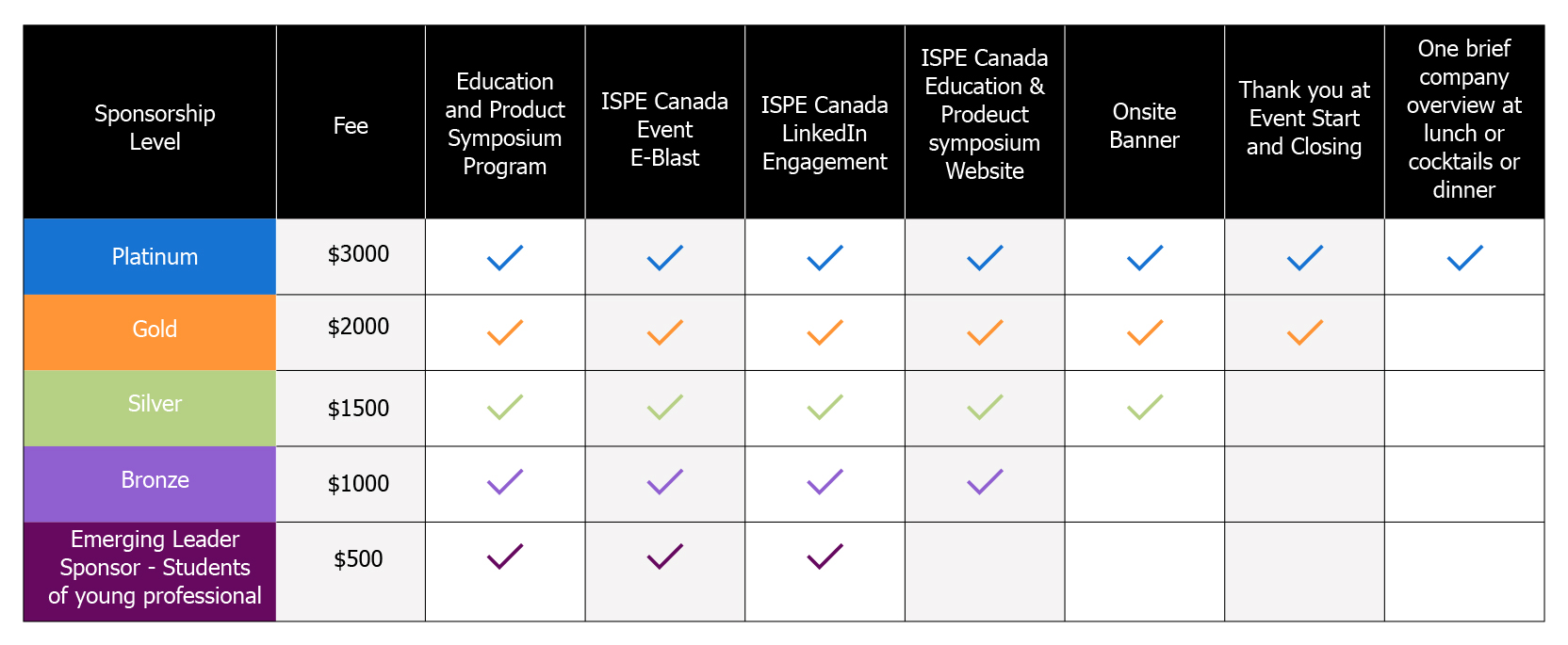
ISPE Canada Education and Product Symposium - May 8 - 9, 2023
ISPE Canada Education and Product Symposium - May 8 - 9, 2023
FOR OTHER SPONSOR OPPORTUNITIES, PLEASE CONTACT
Dina Iezzi: 647-234-3395
ISPE Canada Education and Product Symposium - May 8 - 9, 2023
Attendee Registration – Education and Product Symposium 2023
ISPE Canada Education and Product Symposium - May 8 - 9, 2023
ISPE Canada Education and Product Symposium - May 8 - 9, 2023
ISPE Canada Education and Product Symposium - May 8 - 9, 2023
ISPE Canada Education and Product Symposium - May 8 - 9, 2023
ISPE CANADA AFFILIATE IN-PERSON EDUCATION AND PRODUCT SYMPOSIUM
ISPE Canada Education and Product Symposium - May 8 - 9, 2023






































